Elon Musk's Neuralink
Elon Musk's Neuralink showed off its first human patient. Here are 6 brain chip rivals
While the most well-known, Neuralink is not the only company working on brain chips

Elon Musk’s Neuralink dazzled supporters this week when it revealed the first human patient to be implanted with its first product, in a video livestream of the perso moving a mouse and playing computer chess through a brain implant.
ADVERTISEMENT
Noland Arbaugh, 29, is the the first person implanted with Neuralink’s “Telepathy,” a so-called brain-computer interface (BCI) that looks to help patients with severe paralysis control external technology — like a laptop or smartphone — using only neural signals. Arbaugh said he was released from the hospital the day after the procedure and has since been able to do things he wasn’t previously able to, including playing online chess and the video game Civilization VI.
“It’s not perfect, I would say that we have run into some issues,” Arbaugh said during the livestream on X, Musk’s social media site. “I don’t want people to think that this is the end of the journey, there’s still a lot of work to be done, but it has already changed my life.”
Musk later announced that Neuralink would also work on “Blindsight,” a product he said could cure blindness by beaming “direct vision to the brain.”
He added in another post that Blindsight has been tested and is working in monkeys, further claiming that “no monkey has died or been seriously injured” by a Neuralink device. More than a thousand animals were killed in the course of the startup’s rushed trials, which led to accusations of “grotesque” animal abuse and a lawsuit from a physicians group.
While Musk’s Neuralink is, by far, the most well-known BCI startup, it’s by no means the only one out there — and definitely not the first. Scientists have been studying BCI technology for decades, and dozens of people have been implanted with the brain chips.
Here are six of Neuralink’s brain chip rivals.
ADVERTISEMENT
2
/
8
List slides
Precision Neuroscience
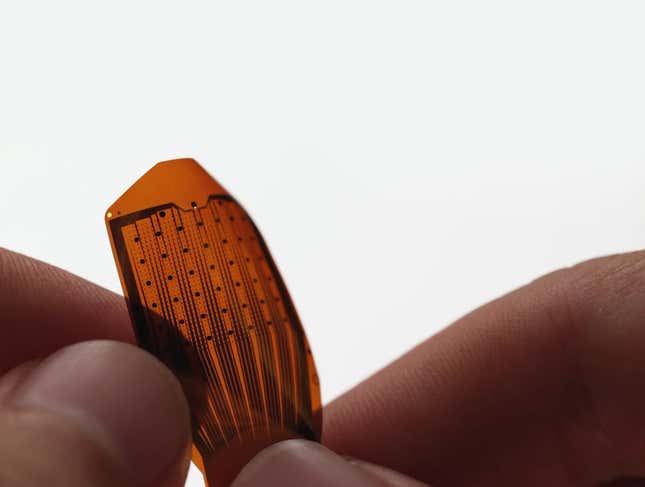 Image: Precision Neuroscience
Image: Precision Neuroscience
Precision Neuroscience is the newest company on this list and the closest to Elon Musk. The New York-based firm was co-founded in 2021 by Benjamin Rapoport, one of the eight founders of Neuralink. Precision in January announced a $41 million Series B funding round.
The firm’s BCI system is the Layer 7 Cortical Interface, an electrode array resembling a piece of scotch tape. Precision aims to help patients with degenerative diseases, such as ALS, regain the ability to communicate and access social media with their minds.
The firm began its first clinical trial in June 2023, implanting its BCI in the brains of three patients who were already in surgery to get tumors removed. Like other BCI companies, Precision will have to work closely with regulators to conduct further testing as it chases approval from U.S. regulators.
“It was incredibly surreal,” Precision President Craig Mermel told CNBC last June. “The nature of the data and our ability to visualize that, you know, I got … chills.”
ADVERTISEMENT
3
/
8
List slides
Blackrock (no, not the investment firm)
 Photo: Blackrock Neurotech/PRNewswire
Photo: Blackrock Neurotech/PRNewswire
Blackrock Neurotech — which has no connection to the investment firm BlackRock — launched in 2008 with the goal of treating physical paralysis, blindness, deafness, and depression. The Salt Lake City-based firm uses an implantable microchip that has 96 arrays, or small needle-shaped brain chips, that detect electrical signals generated by the individual’s thoughts.
ADVERTISEMENT
Those signals are decoded and translated into digital commands. Blackrock has implanted its chips in more than 30 people.
“Our implantable arrays have enabled people to connect directly to computers, control robotic arms and wheelchairs, play video games, even regain sensation – with just their brain signals,” Blackrock co-founder Marcus Gerhardt told The Daily Mail in April 2023.
The company’s brain-computer interface has been studied in patients with paralysis since 2004, and its first-ever BCI product for home use is expected to be released this year. The U.S. Food and Drug Administration has given Blackrock the “Breakthrough Device” designation for its home use BCI, MoveAgain.
Blackrock’s BCI technology is also the first to be featured in an art exhibit. The American Association for the Advancement of Science in April 2023 opened a gallery featuring digital art created by patients with Blackrock’s implants using Adobe Photoshop and Microsoft Paint.
ADVERTISEMENT
4
/
8
List slides
Synchron
 Photo: ANGELA WEISS / AFP (Getty Images)
Photo: ANGELA WEISS / AFP (Getty Images)
Brooklyn, New York-based Synchron has developed a brain-computer interface called the Synchron Switch.
ADVERTISEMENT
The device is inserted through the patient’s blood vessels, allowing people with limited mobility to operate some technology — like smart home devices — with their minds. The company’s Stentrode is fitted with tiny sensors and, post-insertion, sits next to the large vein beside the brain’s motor cortex. Unlike other BCIs, Synchron’s technology isn’t very invasive, which may make it more accessible.
The 12-year-old company has implanted six patients in the U.S. and four patients in Australia. However, it will need to carry out additional trials before it can receive regulatory approval and broader commercialization.
In February, Synchron purchased a minority equity stake in Acquandas, which grants it exclusive access to the German manufacturer’s layering technology for medical devices. Synchron is backed by a slew of investors who poured a combined $75 million into the company in December 2022. Those backers include Bill Gates, Jeff Bezos, and yes, Neuralink’s Elon Musk.
ADVERTISEMENT
5
/
8
List slides
Onward Medical
 Photo: Denis Balibouse (Reuters)
Photo: Denis Balibouse (Reuters)
Netherlands-based Ownard Medical was founded in 2014 by neuroscience researchers at the Swiss Federal Institute of Technology, but it now has a growing team in Boston.
ADVERTISEMENT
Earlier this month, the firm was accepted into the Food and Drug Administration’s total product life cycle advisory program for its BCI technology. The initiative was launched by the FDA to provide patients with faster access to medical devices and hasten development. Onward has also recently received the“Breakthrough Device” designation from the FDA for its BCI.
“TAP enables us to reduce the time and cost to deliver the benefits of ARC-BCI to people living with paralysis,” Onward CEO Dave Marver said in a statement.
Onward has developed the ARC-BCI system, which combines BCI with the company’s ARC-IM simulator. The non-invasive technology delivers electric pulses to the spinal cord to restore functionality.
In May 2023, Onward announced its first successful human trial of ARC-IM and that pairing its ARC Therapy with a wireless BCI gave an individual control over when and how he moved his paralyzed legs.
ADVERTISEMENT
6
/
8
List slides
BrainGate
 Photo: Steve Fisch/Stanford Medicine
Photo: Steve Fisch/Stanford Medicine
Braingate launched the first-ever implanted brain-computer interface for human usage in 2004 in conjunction with Cyberkinetics, a firm later acquired by Blackrock Neurotech. Braingate is run by Tufts University, a private school in Medford, Massachusetts, and partnered with several colleges, including Brown University, Emory University, and Stanford University.
ADVERTISEMENT
Braingate’s current device is manufactured by Blackrock; the implant is about the size of a “baby aspirin” and consists of at least two devices, each with 100 thin electrodes that are implanted on the surface of the brain.
The academic group’s first device was implanted into the head of Matthew Nagle. Nagle was paralyzed from the neck down after being stabbed, which severed his spinal cord. He became an exemplary player of the Pong video game using his BCI but died in 2007 after contracting sepsis. Three other patients were given the same implant between 2004 and 2009.
BrainGate is currently running a clinical trial for their second-generation BCI, designed for people with tetraplegia (paralysis in the upper and lower body). The trial was started in 2009 and is expected to end in 2038.
ADVERTISEMENT
7
/
8
List slides
Paradromics
 Photo: Ralf Liebhold (Shutterstock)
Photo: Ralf Liebhold (Shutterstock)
Austin, Texas-based Paradromics was founded in 2015 and is developing its own BCI, the Connexus Direct Data Interface. Like other BCIs, Paradromics implants an array of tiny electrodes directly into the brain tissue. The array measures and translates signals that are sent through a transceiver under the skin in the chest and through to an external device.
ADVERTISEMENT
The BCI will require invasive brain surgery, like Neuralink’s technology, and last for about 10 years, CEO Matt Angle told CNBC last May after the company received the “Breakthrough Device” designation from FDA. Paradromics raised $33 million from investors last year, including the Westcott Investment Group and Dolby Family Ventures.
Correction: A an earlier version of this article incorrectly compared Paradromic’s technology to Synchron’s brain computer interface. Synchron’s BCI does not require invasive brain surgery, while Paradromic’s does.
ADVERTISEMENT
Elon Musk says Neuralink's first human patient can move a computer mouse with their mind
The brain chip startup performed its first implant in a human last month
Elon Musk says his brain chip company Neuralink actually put one in a human
The billionaire says the patient who underwent the startup's experimental surgery is "recovering well"
Of course Elon Musk's first human Neuralink patient is playing hands-free Mario Kart
"This is going to change the world," the patient said
Neuralink’s ‘hack job’ brain chip trials aren’t fit for humans, congressman tells FDA
Elon Musk's biotech company had numerous safety lapses, Rep. Earl Blumenauer wrote in a letter.