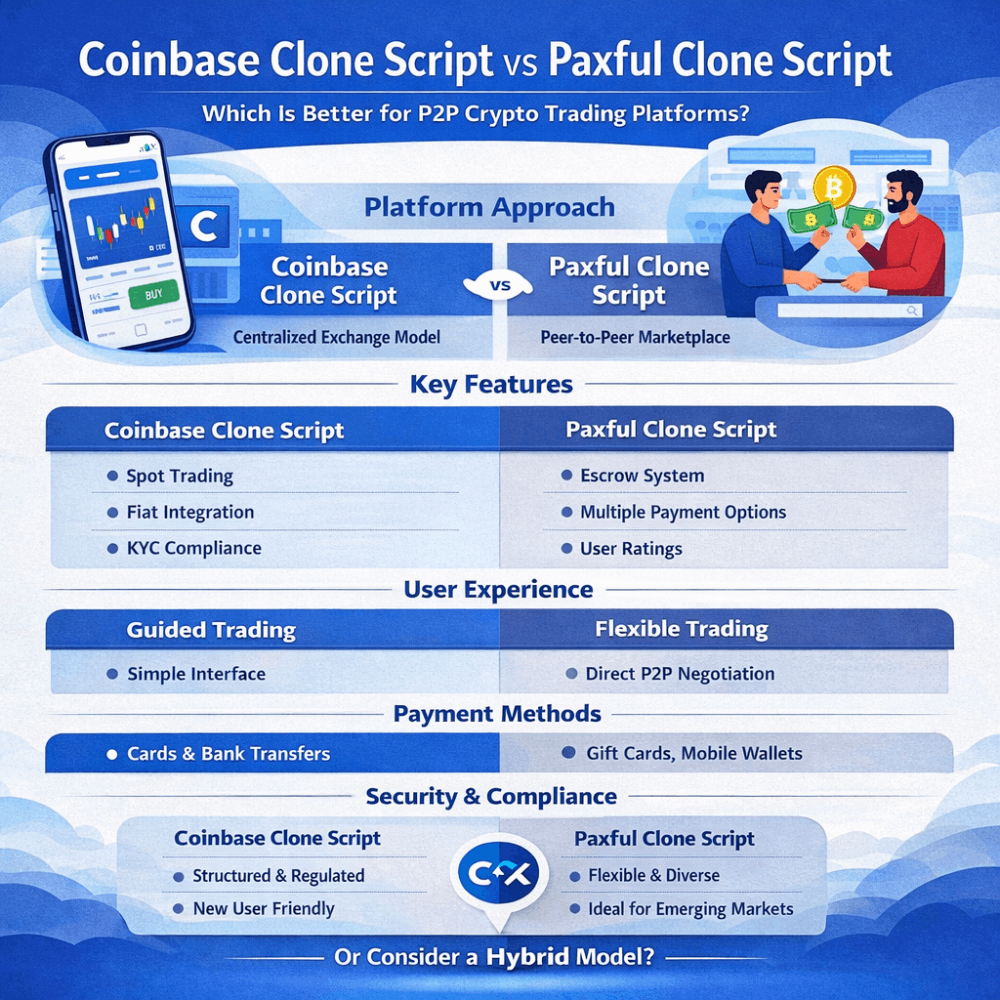
What is activation energy?

Activation energy represents this energy barrier. Before the reaction begins, the kinetic energy of the reactants must be increased to a certain level. This is the amount of energy required to position the reactant molecules correctly and weaken their bonds. Activation energy is the minimum amount of energy required for the reactants to reach higher energy levels at the beginning of the reaction.
Activation energy is an important factor that determines the reaction rate. A higher activation energy can cause the reaction to occur more slowly because the reactant molecules require more energy to overcome this energy barrier. Reducing the activation energy can increase the rate of the reaction and cause it to occur more quickly.
Activation energy is an important thermodynamic concept that affects the rate and efficiency of a reaction. It is important for understanding the rate and mechanism of chemical reactions.
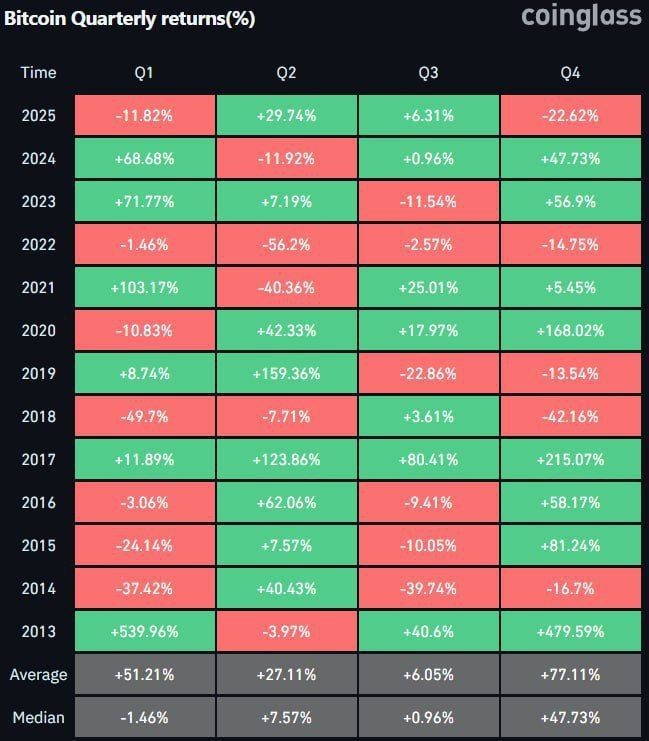
The activation energy of a chemical reaction is often shown in potential energy diagrams. This diagram graphically shows the energy change as the reaction progresses.
The activation energy of a reaction is represented as an energy peak as the reaction proceeds. This is the highest energy level that reactant molecules must pass through before the reaction can be initiated. Activation energy is a key factor determining the reaction rate because the reaction rate depends on the rate at which it overcomes this energy barrier.
In some reactions, catalysts can increase the reaction rate by reducing the activation energy. Catalysts can reduce the activation energy by changing the reaction mechanism or by creating temporary intermediates. This can lead to the reaction occurring faster and lower temperature and energy requirements.
Activation energy is important for understanding the rate, efficiency and thermodynamic stability of chemical reactions. It is a basic thermodynamic concept that includes the initial conditions of the reaction and many factors that affect the reaction rate.
Activation energy refers to the minimum energy level at which the bonds between molecules must be broken and new bonds must be formed in order for a chemical reaction to occur. Therefore, reactant molecules must overcome this energy barrier before a reaction can begin. However, it is rare for reactant molecules to naturally have this energy. Therefore, external factors such as heating or catalyst are often required.
Activation energy determines the kinetics of chemical reactions. That is, the reaction rate is directly related to the activation energy required for the reaction to start. A higher activation energy causes the reaction to occur at a slower rate, while a lower activation energy causes the reaction to occur faster.
To increase the reaction rate, the amount of energy required to overcome the activation energy can be reduced. This can be achieved through catalysts used to increase the rate at which the reaction occurs. Catalysts can reduce the activation energy by providing an alternative pathway for the reaction or by changing the reaction mechanism, allowing the reaction to occur more quickly.
Consequently, activation energy refers to the minimum amount of energy required for chemical reactions to begin. This energy barrier can be thought of as the energy threshold at which molecular bonds must be broken and new bonds must be formed in order for the reaction to occur. This concept helps us deepen our understanding of the kinetics and mechanism of chemical reactions.